lattice energy of kcl
The lattice energies for NaCl most often quoted in other texts is about 765 kJmol. 816 rows Lattice energy calculated kJmol Lattice energy measured in Born-Haber-Fajan cycle.
 |
| Solved The Lattice Energy Of Potassium Chloride Is The Chegg Com |
Between CaO and SrO the magnitude of the lattice energy of SrO is lower due to the larger ionic radius of the strontium ion relative to the calcium ion.

. Therefore The order of lattice energy is NaF NaCl KCl RbCl. Both sign conventions are widely used. Order of increasing magnitude of lattice. In the case of this ionic molecule the lattice energy is the energy required for the following reaction to proceed.
KCl Solution The correct option is D M gO Lattice energy depends on two important factors. Size of the ion On comparing the four given compounds we. The larger the lattice. The lattice energy of KCl is -715 kJmol and the enthalpy of.
Add a Comment Study guides Chemistry 20 cards How does a buffer work What. Given Here Enthalpy of sublimation of Potassium 890 kJmol. The lattice energies of KFKClKBr and KI follows the order. Ionization Energy for Potassium IEK 419 kJmol.
The important consequence of the lattice energies are. Lattice energyHeat of formation-Heat of atomization- Dissociation energy- Sum of Ionization energies- Sum of Electron affinities Or Lattice Energy LE -411- 107 122 496 349. Calculate the Lattice Energy of KCl s given the following data using the Born-Haber cycle. 4 rows In general the lattice energy tends to increase in a period.
Youll get a detailed solution from a subject matter expert that helps you learn core concepts. The Lattice energy of KCl is -717 kJmol. Hence the correct option is C. The lattice energy is the energy required to separate one mole of a solid ionic compound into its gaseous ions.
Following this convention the lattice energy of NaCl would be 786 kJmol. NaCl has the highest melting point both. ΔHsublimation K 792 kJmol IE1 K 4187 kJmol Bond EnergyClCl 2428. The relationship between the lattice energy and the lattice.
The lattice energies of KF KCl KBr and KI follow the order. Wiki User 2014-10-15 191805 This answer is. Charge on the ion 2. A KFKClKBrKI B KIKBrKCLKF C KFKClKIBr D KIKBrKFKCl Medium Solution Verified by Toppr.
Lattice energy is also called lattice enthalpy. This problem has been solved. 4 rows For KCL the melting point is about 1418oF or 770oC and its lattice energy is about 715. Basically saying that the two ions have a mismatched size and resulting polarizability that changes the lattice energy of the whole dern thing.
NaCls Na g Cl g Here the energy that must be supplied to 1. The lattice energy for KCl is 715 kJ mol-1. Compare with the method shown below Lattice Energy is Related to Crystal Structure There. The lattice energy of NaCl for.
I The greater the lattice.
 |
| Solved Which Compound In Each Of The Following Pairs Of Ionic Substances Has The Most Exothermic Lattice Energy Justify Your Answers A Nacl Kcl D Fe Oh 2 Fe Oh 3 B Lif Licl E |
 |
| Lattice Energy Born Haber Cycle Examples Expii |
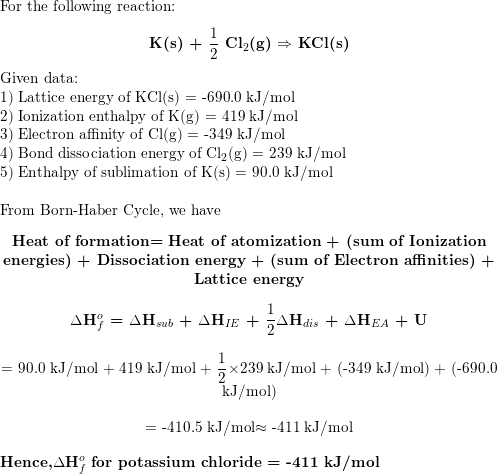 |
| Use The Following Data To Estimate Delta H F Ci Quizlet |
 |
| Solved Use The Following Information To Calculate The Heat Of Sublimation For Potassium K Heat Of Formation For Kcl S 437 Kj Mol Electron Affinity For Cl 349 Kj Mol Ionization Energy For |
 |
| Solved Use The Born Haber Cycle And Data From Appendix Ivb And Chapters 4 And 10 To Calculate The Lattice Energy Of Kcl Dh Sub For Potassium Is 89 0 Kj Mol |
Posting Komentar untuk "lattice energy of kcl"